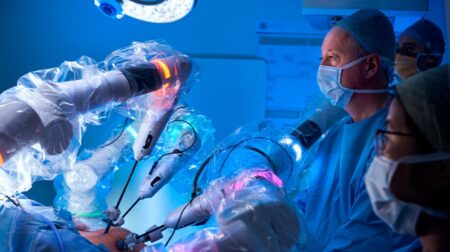
Medical device company Lightpoint Medical has received CE Mark approval for its robotic gamma probe Sensei, which can assist in intra-operative cancer detection.
The miniaturised surgical tool has been designed for detection of sentinel lymph nodes as well as cancer metastasis through the lymphatic system.
Lightpoint said its technology will enable more precise, targeted cancer surgery and is applicable across a wide range of major cancer types, including lung, colorectal, stomach, gynecological, and prostate cancer.
Dr David Tuch, CEO of Lightpoint Medical, said: “Sensi promises accurate real-time cancer detection for robot-assisted cancer surgery. Currently, surgeons have no way to precisely detect cancer intra-operatively. As a result, millions of patients suffer every year as cancer is frequently left behind or healthy, functional tissue is needlessly removed.”
The device was successfully registered with the US Food and Drug Administration in September 2020 and will now be commercially available in European hospitals.
Robotic surgeon, Dr Jim Adshead, based at Lister Hospital, East and North Herts NHS Trust, said he is eager to start using the tool in surgery.
“I have been closely involved in the development of the technology since early usability testing and I’m extremely hopeful for its potential in advancing the treatment of prostate cancer,” said Adshead.