Med tech start-up Qure.ai has been awarded £3.2m from the UK government’s Small Business Research Initiative (SBRI) Healthcare programme to further develop its early detection of lung cancer system.
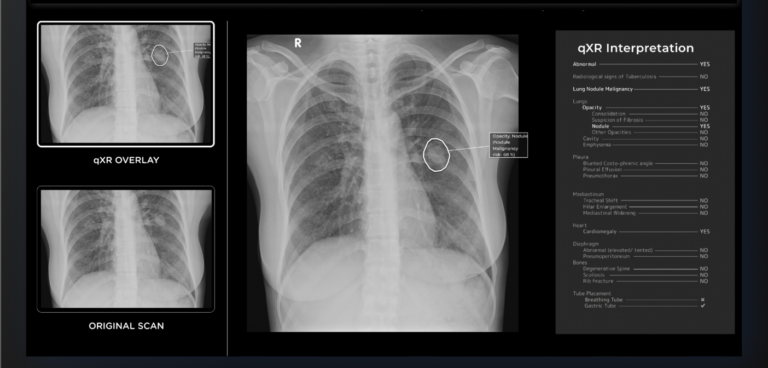
Qure.ai’s AI-powered chest X-ray interpretation solution, which is CE-approved, uses millions of images and deep learning. It classifies chest X-rays as normal or abnormal, identifies abnormal findings, and highlights them on the X-ray.
Furthermore, it can detect and localise multiple findings in a chest X-ray including issues with the lungs, pleura, heart, bones, and diaphragm. The qXR algorithm detects lung nodules and assigns them a lung malignancy-risk score, reducing the chances of missed diagnosis.
With this SBRI funding, Qure.ai aims to demonstrate real-world evidence of AI-aided diagnosis of lung cancer based on triage of suspected lung nodules on chest X-rays. The company added its system enables immediate reporting for prompt follow through and confirmation.
This will lead to faster chest computed tomography (CT) scans for suspected cases and eventually impact early-stage lung cancer detection, the team said.
Prashant Warier, CEO and co-founder, Qure.ai, said: “Lung cancer is the leading cause of death in the U.K. Its aggressive nature and absence of early, visible signs often leads to diagnostic and treatment delays and poor outcomes.
“The five-year survival rates remain low at roughly 14%, with 30% of patients dying within 90 days after diagnosis. Identifying it at earlier stages impacts survival rates positively and allows for the ability to use less toxic treatment or intervention. However, this is difficult because of varying protocols and immense workloads. The use of AI technologies can help in early detection of potentially cancerous nodules from chest X-rays.”
Qure.ai will collaborate with leading NHS Trusts that have a focus on lung cancer including the Nottingham University Hospital Trust, The Royal Marsden NHS Trust, University College London Hospital NHS Trust, University Hospitals Birmingham NHS Trust, and several other regional NHS Trusts.
Clinical and cost-effectiveness data will be used in a prospective evaluation to establish clinical relevance before moving to wider adoption.